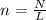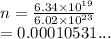Chemistry, 06.01.2021 17:40 nelyanariba981p555ve
How many moles are in the following atoms? 6.34 x 1019 molecules of ammonium nitrate

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

You know the right answer?
How many moles are in the following atoms? 6.34 x 1019 molecules of ammonium nitrate...
Questions

Social Studies, 10.12.2020 23:20


Mathematics, 10.12.2020 23:20



Mathematics, 10.12.2020 23:20


English, 10.12.2020 23:20


Spanish, 10.12.2020 23:20


Mathematics, 10.12.2020 23:20

Mathematics, 10.12.2020 23:20




History, 10.12.2020 23:20

Health, 10.12.2020 23:20

Computers and Technology, 10.12.2020 23:20

Social Studies, 10.12.2020 23:20