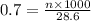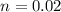Chemistry, 05.01.2021 16:50 highflylex279
You have a 0.7 M solution. Your job is to produce 50 mL of a 0.4 M solution.
A. How much of the 0.7 M solution do you need to start with? (Show your work.)
B. How many moles of solute were in the 0.7 M solution? (Show your work.)
C. How much water do you need to add to the previous amount of the 0.7 M solution to dilute it to
0.4 M? (Show your work.)
D. A student decides to put double the amount of water calculated in Part C. Describe what effect this
will have on the overall concentration of the resulting solution. Justify your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3


Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
You have a 0.7 M solution. Your job is to produce 50 mL of a 0.4 M solution.
A. How much of the 0.7...
Questions

Social Studies, 29.08.2020 21:01


Mathematics, 29.08.2020 21:01






Mathematics, 29.08.2020 21:01






Spanish, 29.08.2020 21:01

History, 29.08.2020 21:01




Computers and Technology, 29.08.2020 21:01


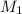 = molarity of stock solution = 0.7 M
= molarity of stock solution = 0.7 M
 = volume of stock solution = ?
= volume of stock solution = ?
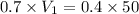


 = volume of solution in ml
= volume of solution in ml