
Chemistry, 05.01.2021 16:40 Skylar4483
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
of CO2?
2C2H2(g) + 502(g) → 2H2O(g) + 4CO2(g)
A. 20.0L
B. 44.8L
C. 80.0L
D. 100 L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1


Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
Questions

Mathematics, 15.05.2021 01:00

Business, 15.05.2021 01:00

Mathematics, 15.05.2021 01:00


Health, 15.05.2021 01:00



Chemistry, 15.05.2021 01:00

SAT, 15.05.2021 01:00


Arts, 15.05.2021 01:00


Mathematics, 15.05.2021 01:00



Spanish, 15.05.2021 01:00

English, 15.05.2021 01:00


Physics, 15.05.2021 01:00

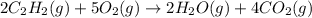
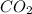 is formed by = 2 L of
is formed by = 2 L of 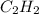
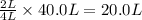 of
of 

