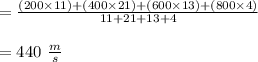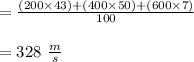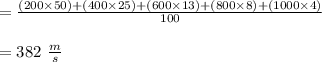Suppose a sample of gas is composed of 100 molecules, with speeds given below. Speed (m/s) Number 200 23 400 42 600 26 800 8 1000 1 According to the kinetic molecular theory, if the absolute temperature of the gas is halved, what is a reasonable estimation of the new distribution of speeds of the molecules

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Suppose a sample of gas is composed of 100 molecules, with speeds given below. Speed (m/s) Number 20...
Questions


History, 21.06.2019 20:30


Social Studies, 21.06.2019 20:30




World Languages, 21.06.2019 20:30

Mathematics, 21.06.2019 20:30

English, 21.06.2019 20:30



Mathematics, 21.06.2019 20:30



English, 21.06.2019 20:30

Chemistry, 21.06.2019 20:30