Chemistry, 05.01.2021 16:00 AquaTyrant5054
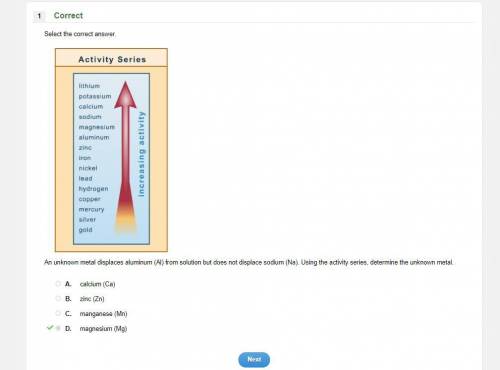
An unknown metal displaces aluminum (Al) from solution but does not displace sodium (Na). Using the activity series, determine the unknown metal.
A.
calcium (Ca)
B.
zinc (Zn)
C.
manganese (Mn)
D.
magnesium (Mg)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1


Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3
You know the right answer?
An unknown metal displaces aluminum (Al) from solution but does not displace sodium (Na). Using the...
Questions



History, 05.03.2021 21:40

Mathematics, 05.03.2021 21:40



Mathematics, 05.03.2021 21:40


Mathematics, 05.03.2021 21:40

Mathematics, 05.03.2021 21:40




History, 05.03.2021 21:40


History, 05.03.2021 21:40

Spanish, 05.03.2021 21:40

Biology, 05.03.2021 21:40

Social Studies, 05.03.2021 21:40