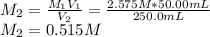Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
Asolution is prepared by diluting 50.00 ml of 2.575 m solution of hno3 to 250.0 ml. what is the mola...
Questions

Mathematics, 10.02.2021 02:30


Mathematics, 10.02.2021 02:30

Chemistry, 10.02.2021 02:30


Mathematics, 10.02.2021 02:30


Mathematics, 10.02.2021 02:30


Mathematics, 10.02.2021 02:30

Advanced Placement (AP), 10.02.2021 02:30

Mathematics, 10.02.2021 02:30

Spanish, 10.02.2021 02:30

Mathematics, 10.02.2021 02:30

Social Studies, 10.02.2021 02:30

Social Studies, 10.02.2021 02:30

Mathematics, 10.02.2021 02:30



English, 10.02.2021 02:30

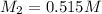
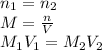
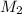 , one obtains:
, one obtains: