

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
4. (05.06 LC)
The actual yield of a product in a reaction was measured as 2.80 g. If the theoretica...
Questions

Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

Physics, 11.10.2020 14:01

Geography, 11.10.2020 14:01


English, 11.10.2020 14:01

Chemistry, 11.10.2020 14:01

Arts, 11.10.2020 14:01



Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

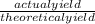 ×100). So, simply put 2.80 over 3.12 and whatever you get multiply times 100 to make it a percentage.
×100). So, simply put 2.80 over 3.12 and whatever you get multiply times 100 to make it a percentage. 

