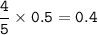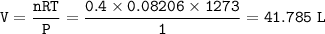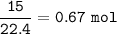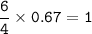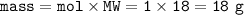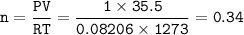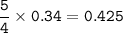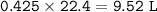Catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (HNO3) on a commercial scale.
The products are produced at 1000°C (1273 K) and at at-
mospheric pressure.
4 NH; (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (1)
a. What volume of NO is produced in the reaction vessel
by the reaction of 0.500 mol O2?
b. What mass of H2O is produced by the reaction of 15.0 L
of NH3?
c. How many liters of O, must react to produce 35.5 L of
NO?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (H...
preparation of nitric acid (H...
Questions

Mathematics, 27.08.2019 10:30



History, 27.08.2019 10:30



Mathematics, 27.08.2019 10:30


Mathematics, 27.08.2019 10:30

Chemistry, 27.08.2019 10:30

Mathematics, 27.08.2019 10:30

Health, 27.08.2019 10:30




History, 27.08.2019 10:30


Mathematics, 27.08.2019 10:30


History, 27.08.2019 10:30