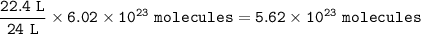Chemistry, 03.01.2021 01:00 jesuscruzm2020
You have two containers at 25°C and 1 atm. One has 22.4 L of hydrogen gas, and the other has 22.4 L of oxygen gas.
Which statement is true?
The hydrogen gas container has more molecules than the oxygen gas container.
The oxygen gas container has more molecules than the hydrogen gas container.
Both containers have the same number of molecules.
Both containers contain 6.022×1023 molecules of gas.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

You know the right answer?
You have two containers at 25°C and 1 atm. One has 22.4 L of hydrogen gas, and the other has 22.4 L...
Questions

Social Studies, 08.12.2021 05:30


Chemistry, 08.12.2021 05:30

Biology, 08.12.2021 05:30


History, 08.12.2021 05:30


Mathematics, 08.12.2021 05:30


Mathematics, 08.12.2021 05:30





Computers and Technology, 08.12.2021 05:30


Mathematics, 08.12.2021 05:30


Mathematics, 08.12.2021 05:30

Social Studies, 08.12.2021 05:30