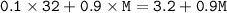Chemistry, 02.01.2021 20:30 famousshorty
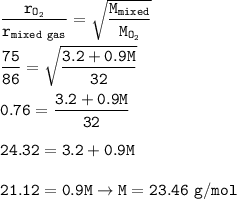
A vessel of volume 100ml contains 10% of oxygen and 90% of an unknown gas. The gases diffuses in 86 second through a small hole of vessel.
If pure oxygen under similar
conditions and diffuses in 75 second, find the molecular weight of unknown gas?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 09:00
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3
You know the right answer?
A vessel of volume 100ml contains 10% of oxygen and 90% of an unknown gas. The gases diffuses in 86...
Questions





Mathematics, 26.08.2021 18:10



Mathematics, 26.08.2021 18:10


Mathematics, 26.08.2021 18:10


Law, 26.08.2021 18:10





Mathematics, 26.08.2021 18:10

French, 26.08.2021 18:10


Mathematics, 26.08.2021 18:10