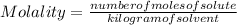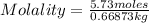Chemistry, 02.01.2021 16:30 jessica2138
Calculate the molality of a 5.73 M ethanol (C2H5OH) solution whose density is 0.9327 g/mL.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1
You know the right answer?
Calculate the molality of a 5.73 M ethanol (C2H5OH) solution whose density is 0.9327 g/mL....
Questions

Biology, 29.09.2020 20:01



Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01


History, 29.09.2020 20:01

History, 29.09.2020 20:01

History, 29.09.2020 20:01

Social Studies, 29.09.2020 20:01

Social Studies, 29.09.2020 20:01


Chemistry, 29.09.2020 20:01



Biology, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01

Mathematics, 29.09.2020 20:01

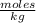 .
.