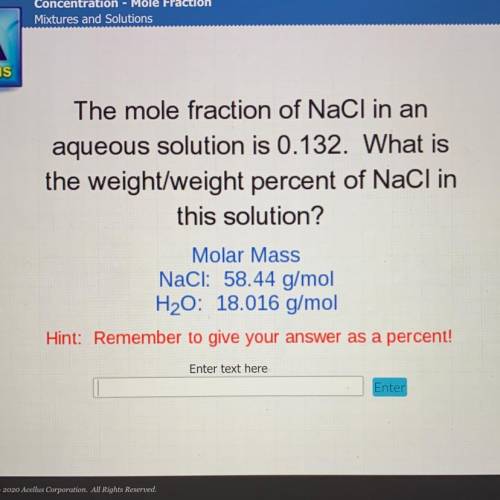
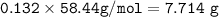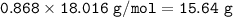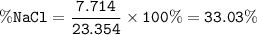The mole fraction of NaCl in an
aqueous solution is 0.132. What is
the weight/weight percent...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1


Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
Questions

Social Studies, 18.03.2022 08:10






SAT, 18.03.2022 08:10


Mathematics, 18.03.2022 08:10

English, 18.03.2022 08:10

Chemistry, 18.03.2022 08:10

History, 18.03.2022 08:10

English, 18.03.2022 08:10


French, 18.03.2022 08:10




Mathematics, 18.03.2022 08:10