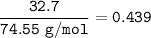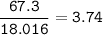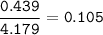An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in...

Chemistry, 31.12.2020 23:40 Chandler1Gaming
An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in
this aqueous solution?
Molar Mass
KCI: 74.55 g/mol
H2O: 18.016 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Questions

Mathematics, 19.01.2021 21:10

Computers and Technology, 19.01.2021 21:10

Mathematics, 19.01.2021 21:10

English, 19.01.2021 21:10


Chemistry, 19.01.2021 21:10

Computers and Technology, 19.01.2021 21:10


Mathematics, 19.01.2021 21:10






Computers and Technology, 19.01.2021 21:10


Mathematics, 19.01.2021 21:10

Mathematics, 19.01.2021 21:10

Mathematics, 19.01.2021 21:10

Mathematics, 19.01.2021 21:10