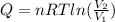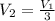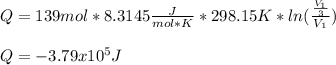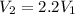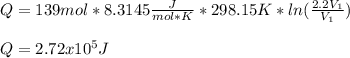An ideal gas resides in a closed cylinder (diameter is 0.5 ft) with a frictionless piston. The initial conditions are 139 mol of the ideal gas at 25°C. The piston is compressed isothermally to one third of the initial volume. The heat capacity C_v of an ideal gas is 1.5 middot R.
Required:
a. What is the heat interaction (kJ) for this process?
b. The piston now expands isothermally to 120% of the same initial volume. Will the heat interaction increase, decrease, or stay the same?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1


You know the right answer?
An ideal gas resides in a closed cylinder (diameter is 0.5 ft) with a frictionless piston. The initi...
Questions



Chemistry, 20.09.2020 20:01

Mathematics, 20.09.2020 20:01


Chemistry, 20.09.2020 20:01


Chemistry, 20.09.2020 20:01



Mathematics, 20.09.2020 20:01





Mathematics, 20.09.2020 20:01

Physics, 20.09.2020 20:01

English, 20.09.2020 20:01

Biology, 20.09.2020 20:01

English, 20.09.2020 20:01