temperature will change from

Chemistry, 31.12.2020 02:20 katelynalivia
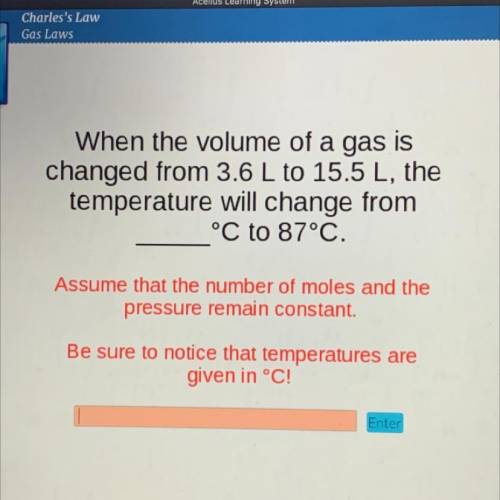
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
°C to 87°C.
Assume that the number of moles and the
pressure remain constant.
Be sure to notice that temperatures are
given in °C!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3

Chemistry, 23.06.2019 17:30
Insoluble substances can dissolve in all solvents. true or false
Answers: 2
You know the right answer?
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
temperature will change from
Questions





History, 04.07.2019 13:40

English, 04.07.2019 13:40





English, 04.07.2019 13:40



Mathematics, 04.07.2019 13:40

Spanish, 04.07.2019 13:40

History, 04.07.2019 13:40

Social Studies, 04.07.2019 13:40



Chemistry, 04.07.2019 13:40



