Chemistry, 31.12.2020 01:00 ldelgado97
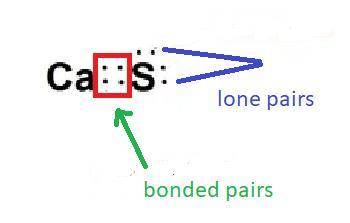
In a molecule of calcium sulfide, calcium has two valence electron bonds, and a sulfur atom has six valence electrons. How many lone pairs of electrons are present in the Lewis structure of calcium sulfide? Also, state why.
A. one
B. two
C. three
D. four
E. none

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
In a molecule of calcium sulfide, calcium has two valence electron bonds, and a sulfur atom has six...
Questions

History, 12.07.2019 17:20



English, 12.07.2019 17:20

Mathematics, 12.07.2019 17:20



Mathematics, 12.07.2019 17:20

Biology, 12.07.2019 17:20


Social Studies, 12.07.2019 17:20



Mathematics, 12.07.2019 17:20





Social Studies, 12.07.2019 17:20

Mathematics, 12.07.2019 17:20