
Chemistry, 29.12.2020 22:00 jessicaaaamartin
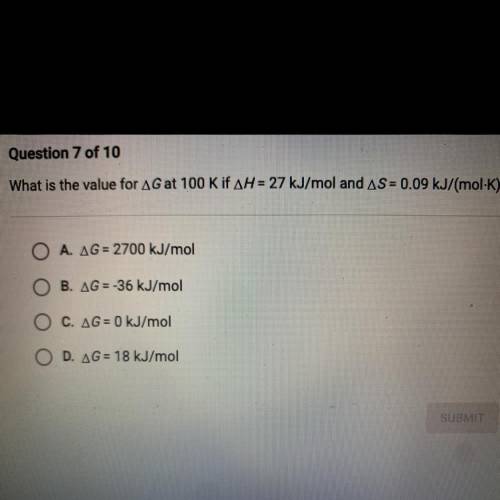
What is the value for AG at 100 K if AH = 27 kJ/mol and AS = 0.09 kJ/(mol-K)?
A. AG= 2700 kJ/mol
B. AG = -36 kJ/mol
C. AG = 0 kJ/mol
D. AG = 18 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2


Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
What is the value for AG at 100 K if AH = 27 kJ/mol and AS = 0.09 kJ/(mol-K)?
A. AG= 2700 kJ/mol
Questions





History, 11.04.2020 03:02

Mathematics, 11.04.2020 03:02




World Languages, 11.04.2020 03:02








Mathematics, 11.04.2020 03:02


Mathematics, 11.04.2020 03:02



