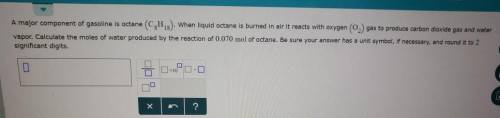
A major component of gasoline is octane . When liquid octane is burned in air it reacts with oxygen gas to produce carbon dioxide gas and water vapor. Calculate the moles of water produced by the reaction of of octane. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
A major component of gasoline is octane . When liquid octane is burned in air it reacts with oxygen...
Questions




Biology, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Social Studies, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Advanced Placement (AP), 18.03.2021 01:30


Mathematics, 18.03.2021 01:30


Geography, 18.03.2021 01:30





English, 18.03.2021 01:30