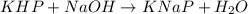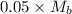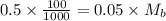Chemistry, 28.12.2020 15:50 zfghiiooi5
Someone help me please im stuck on a equation
3. Rearrange the titration calculation to find the Mb in a solution. (* Mb = molarity of the basic solution )
(~0.3 mL) of indicator to the KHP
solution.
(~ 0.5 mL or less) of NaOH
The moles of acid= 0.5 x 100/1000=0.05 Mb = (0.5/55)x1000=0.9

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1


Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
Someone help me please im stuck on a equation
3. Rearrange the titration calculation to find the Mb...
Questions

Mathematics, 10.02.2021 17:30

Mathematics, 10.02.2021 17:30


Social Studies, 10.02.2021 17:30


English, 10.02.2021 17:30






English, 10.02.2021 17:30


Mathematics, 10.02.2021 17:30


Mathematics, 10.02.2021 17:30

Mathematics, 10.02.2021 17:30


Mathematics, 10.02.2021 17:30

History, 10.02.2021 17:30