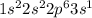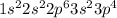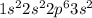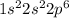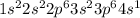Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Give electron configurations for atoms of these elements: na, s, mg, ne, and k....
Questions


Mathematics, 08.01.2021 18:20


Mathematics, 08.01.2021 18:20

Chemistry, 08.01.2021 18:20


Mathematics, 08.01.2021 18:20




Biology, 08.01.2021 18:20

Mathematics, 08.01.2021 18:20

History, 08.01.2021 18:20

Mathematics, 08.01.2021 18:20





Mathematics, 08.01.2021 18:20