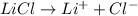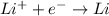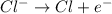Chemistry, 27.12.2020 14:00 saraaaalynn19061
Please help!-- 20 pts!
The electrolyte lithium chloride (LiCl) dissolves into positively charged Li+ and negatively charged Cl– ions in water containing electrodes.
What will happen next?
The positively charged ions will be attracted to the negative electrode and gain electrons. Meanwhile, the negatively charged ions will be attracted to the positive electrode and release electrons.
The negatively charged ions will be attracted to the negative electrode and release electrons. Meanwhile, the positively charged ions will be attracted to the positive electrode and gain electrons.
The positively charged ions will be attracted to the negative electrode and release electrons. Meanwhile, the negatively charged ions will be attracted to the positive electrode and gain electrons.
The negatively charged ions will be attracted to the negative electrode and gain electrons. Meanwhile, the positively charged ions will be attracted to the positive electrode and release electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Please help!-- 20 pts!
The electrolyte lithium chloride (LiCl) dissolves into positively charged Li...
Questions


Computers and Technology, 27.08.2019 03:50


History, 27.08.2019 03:50



Chemistry, 27.08.2019 03:50

History, 27.08.2019 03:50


Chemistry, 27.08.2019 03:50

Computers and Technology, 27.08.2019 03:50




Mathematics, 27.08.2019 03:50



History, 27.08.2019 03:50