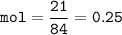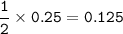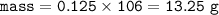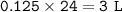Chemistry, 27.12.2020 03:10 makaylapink8167
3. The equation for the decomposition of sodium hydrogencarbonate is :
2 NaHCO3 (s) → Na2CO3 (s) + H2O(l) + CO2(8)
If 21 g of sodium hydrogencarbonate were decomposed, calculate
(a) the mass of the residue formed.
(b) the volume of carbon dioxide evolved measured at r. t.p.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
3. The equation for the decomposition of sodium hydrogencarbonate is :
2 NaHCO3 (s) → Na2CO3 (s) +...
Questions

Mathematics, 24.06.2019 01:00





History, 24.06.2019 01:00


Biology, 24.06.2019 01:00

Social Studies, 24.06.2019 01:00

Mathematics, 24.06.2019 01:00


Mathematics, 24.06.2019 01:00


History, 24.06.2019 01:00




Mathematics, 24.06.2019 01:00