

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 09:30
The earth's surface is (science) a: studied using seismic waves b: constantly changing over time c: only studied indirectly d: the same today as million of years
Answers: 1
You know the right answer?
1.72 moles of NOCI were placed in a 2.50 L reaction chamber
at 427°C. After equilibrium was reached...
Questions






Mathematics, 09.01.2020 22:31

Mathematics, 09.01.2020 22:31

Mathematics, 09.01.2020 22:31


History, 09.01.2020 22:31


Mathematics, 09.01.2020 22:31

Mathematics, 09.01.2020 22:31



Mathematics, 09.01.2020 22:31



History, 09.01.2020 22:31

World Languages, 09.01.2020 22:31

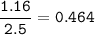
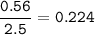
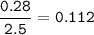
![\tt Kc=\dfrac{[NO]^2[Cl_2]}{[NOCl]^2}\\\\Kc=\dfrac{0.224^2\times 0.112}{0.464^2}=0.026](/tpl/images/1007/8914/ad1b4.png)


