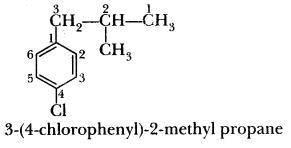Write the structure of the following compound:
=> 3-(4-chlorophenyl)-2-methylpropane...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Questions

Mathematics, 27.10.2021 18:20

English, 27.10.2021 18:20

Mathematics, 27.10.2021 18:20

Spanish, 27.10.2021 18:30

Mathematics, 27.10.2021 18:30


Mathematics, 27.10.2021 18:30

Chemistry, 27.10.2021 18:30

Mathematics, 27.10.2021 18:30



History, 27.10.2021 18:30

Mathematics, 27.10.2021 18:30

Advanced Placement (AP), 27.10.2021 18:30



Advanced Placement (AP), 27.10.2021 18:30


Mathematics, 27.10.2021 18:30