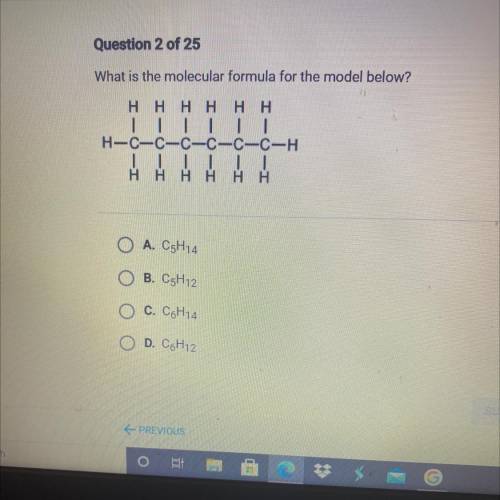
A. C5H14
B. C5H12
C. C6H14
D. C6H12
...

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Questions

Mathematics, 26.06.2020 16:01




Mathematics, 26.06.2020 16:01





Biology, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01



English, 26.06.2020 16:01





Mathematics, 26.06.2020 16:01