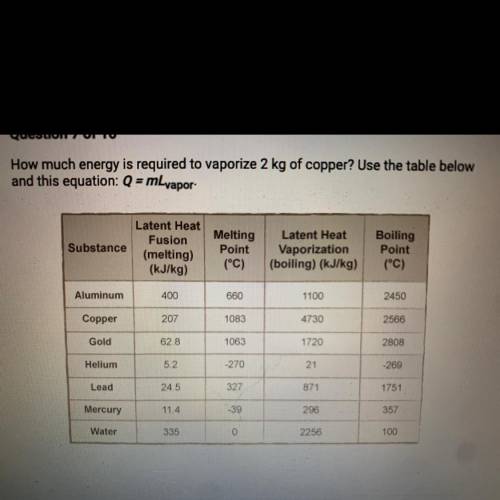
A. 207 kJ
B. 4730 kJ
C. 9460 kJ
D. 414 kJ
...

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1


Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Questions


Mathematics, 06.05.2020 01:24

Computers and Technology, 06.05.2020 01:24

Biology, 06.05.2020 01:24

Social Studies, 06.05.2020 01:24


History, 06.05.2020 01:24


Mathematics, 06.05.2020 01:24


Biology, 06.05.2020 01:24

Biology, 06.05.2020 01:24

Mathematics, 06.05.2020 01:24

English, 06.05.2020 01:24

Mathematics, 06.05.2020 01:24

English, 06.05.2020 01:24

Mathematics, 06.05.2020 01:24

History, 06.05.2020 01:24


Mathematics, 06.05.2020 01:24