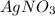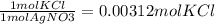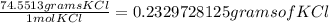Chemistry, 23.12.2020 17:40 biaxialpower789
11. Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride:
KCl(aq) + AgNO3(aq) → AgCl(s) + KNO3(aq)
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion. How many grams of solid KCl must be added to 25.0 mL of 0.125 M AgNO3 solution to completely precipitate the silver?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
11. Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride:
KCl...
Questions


History, 27.07.2019 09:50

Mathematics, 27.07.2019 09:50

English, 27.07.2019 09:50

Mathematics, 27.07.2019 09:50

Mathematics, 27.07.2019 09:50


Chemistry, 27.07.2019 09:50






Social Studies, 27.07.2019 09:50


Mathematics, 27.07.2019 09:50


History, 27.07.2019 09:50

Mathematics, 27.07.2019 09:50

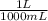 ) = 0.025 L
) = 0.025 L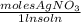 ) = 0.003125 moles
) = 0.003125 moles