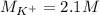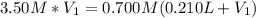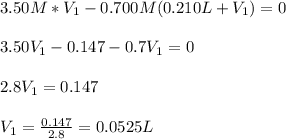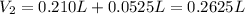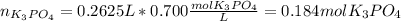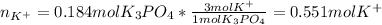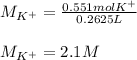Chemistry, 23.12.2020 04:30 neekobecky599
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water to form a 0.700 M K3PO, solution. Calculate the molarity of potassium ions, K in the solution.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water
t...
Questions



History, 03.05.2020 12:49


History, 03.05.2020 12:49



English, 03.05.2020 12:49

Social Studies, 03.05.2020 12:49

Mathematics, 03.05.2020 12:49

English, 03.05.2020 12:49


Mathematics, 03.05.2020 12:49

Mathematics, 03.05.2020 12:49


History, 03.05.2020 12:49

Mathematics, 03.05.2020 12:49


English, 03.05.2020 12:49