
Chemistry, 22.12.2020 19:00 ShawnSaviro4918
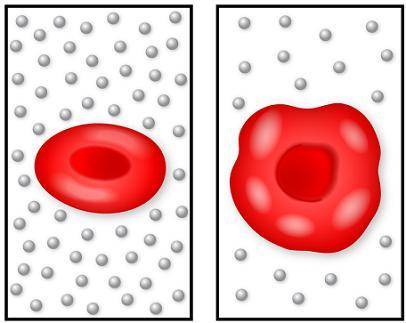
The image on the left shows a normal red blood cell, and the image on the right shows a cell that has been put into a new solution. The circles represent the salt in the solutions.
Which statement describes the motion of the water molecules in this situation?
The water molecules move by active transport into the cell from low water concentration to high water concentration.
The water molecules move by osmosis into the cell from low water concentration to high water concentration.
The water molecules move by osmosis into the cell from high water concentration to low water concentration.
The water molecules move by active transport into the cell from high water concentration to low water concentration.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1

Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2
You know the right answer?
The image on the left shows a normal red blood cell, and the image on the right shows a cell that ha...
Questions

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01



Mathematics, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Business, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Chemistry, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01


History, 18.10.2020 14:01




Biology, 18.10.2020 14:01

Social Studies, 18.10.2020 14:01

Chemistry, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01



