
Chemistry, 22.12.2020 08:40 anaroles04
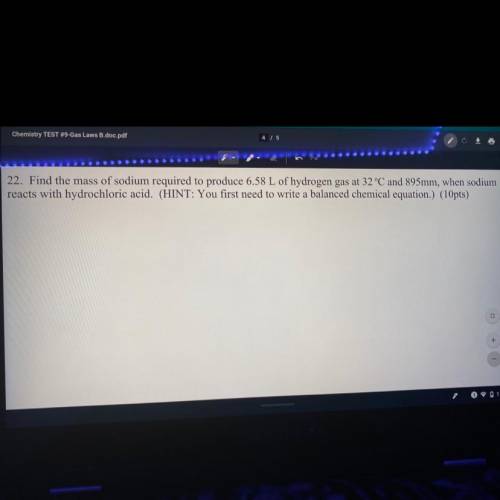
Find the mass of sodium required to reduce 6.58 L of hydrogen gas at 32°C and 895 mm, when sodium reacts with hydrochloric acid. (Hint: you first need to write a balanced chemical equation.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
Find the mass of sodium required to reduce 6.58 L of hydrogen gas at 32°C and 895 mm, when sodium re...
Questions




Computers and Technology, 02.03.2020 23:31

History, 02.03.2020 23:31


Computers and Technology, 02.03.2020 23:31






Computers and Technology, 02.03.2020 23:31


Mathematics, 02.03.2020 23:31

Computers and Technology, 02.03.2020 23:32

Mathematics, 02.03.2020 23:32



Mathematics, 02.03.2020 23:32



