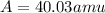Chemistry, 22.12.2020 06:40 91miketaylor
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57.93 amu respectively. What is the atomic mass of these two isotopes? Explain why
the average mass is closer to actual amu of 58.69 for nickel.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57...
Questions

Business, 22.08.2021 18:10

Mathematics, 22.08.2021 18:10

Geography, 22.08.2021 18:10

Mathematics, 22.08.2021 18:10

Business, 22.08.2021 18:10


Mathematics, 22.08.2021 18:20

Business, 22.08.2021 18:20

Physics, 22.08.2021 18:20


English, 22.08.2021 18:20


History, 22.08.2021 18:20



Physics, 22.08.2021 18:20


Chemistry, 22.08.2021 18:20

Chemistry, 22.08.2021 18:20

Advanced Placement (AP), 22.08.2021 18:20

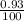
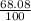
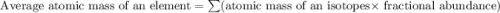
![A=\sum[(63.93)\times \frac{0.93}{100})+(57.93)\times \frac{68.08}{100}]]](/tpl/images/1005/1681/01772.png)