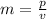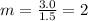Chemistry, 21.12.2020 22:00 hcortez8846
. A marble is rolling at a velocity of 1.5 m/s with a momentum of 3.0 kg×m/s. What is its mass?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2


You know the right answer?
. A marble is rolling at a velocity of 1.5 m/s with a momentum of 3.0 kg×m/s. What is its mass?...
Questions


Mathematics, 23.02.2021 14:00

English, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Biology, 23.02.2021 14:00




Mathematics, 23.02.2021 14:00

Medicine, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Chemistry, 23.02.2021 14:00

Social Studies, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Geography, 23.02.2021 14:00


Spanish, 23.02.2021 14:00