Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
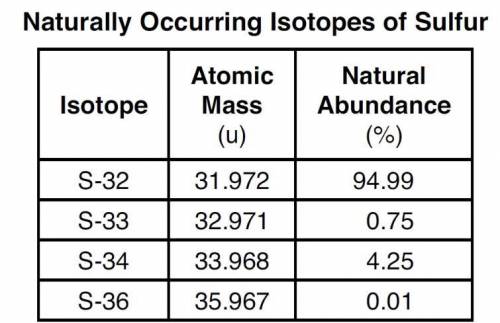
The four naturally occurring isotopes of sulfur are S-32, S-33, S-34, and S-36. The table below show...
Questions



Mathematics, 26.02.2021 01:40

Mathematics, 26.02.2021 01:40

History, 26.02.2021 01:40




Mathematics, 26.02.2021 01:40

Mathematics, 26.02.2021 01:40

History, 26.02.2021 01:40

Mathematics, 26.02.2021 01:40

History, 26.02.2021 01:40

Biology, 26.02.2021 01:40


Mathematics, 26.02.2021 01:40

English, 26.02.2021 01:40



World Languages, 26.02.2021 01:40