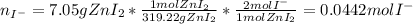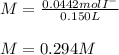Chemistry, 21.12.2020 17:30 aidenbender06
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbonate. Calculate the final molarity of iodide anion in the solution. You can assume the volume of the solution doesn't change when the zinc iodide is dissolved in it. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

You know the right answer?
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbo...
Questions


Arts, 13.01.2021 18:40

Physics, 13.01.2021 18:40

Mathematics, 13.01.2021 18:40

Mathematics, 13.01.2021 18:40



Mathematics, 13.01.2021 18:40


Engineering, 13.01.2021 18:40


Chemistry, 13.01.2021 18:40




Mathematics, 13.01.2021 18:40

Health, 13.01.2021 18:40

Mathematics, 13.01.2021 18:40


Business, 13.01.2021 18:40