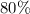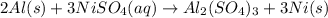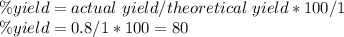Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2


Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Aluminum metal reacts with aqueous nickel(II) sulfate to produce aqueous aluminum sulfate and nickel...
Questions

Social Studies, 28.06.2019 14:30

Mathematics, 28.06.2019 14:30

English, 28.06.2019 14:30

Mathematics, 28.06.2019 14:30






Mathematics, 28.06.2019 14:30


Mathematics, 28.06.2019 14:30

Mathematics, 28.06.2019 14:30


Social Studies, 28.06.2019 14:30


Mathematics, 28.06.2019 14:30

Mathematics, 28.06.2019 14:30


Mathematics, 28.06.2019 14:30