
Chemistry, 19.12.2020 06:00 jsjsjsskakwkowwj
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g)
A) NO increases the rate at which SO3 molecules are formed.
B) NO reacts with SO3 to produce more SO2 molecules.
C) NO decreases collisions between the SO2 and O2 molecules.
D) NO increases the concentration of the SO2 and O2 molecules.
E) NO increases the activation energy of the SO2 and O2 molecules.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1


Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g...
Questions

English, 17.03.2020 22:08

Mathematics, 17.03.2020 22:08






Mathematics, 17.03.2020 22:09

Mathematics, 17.03.2020 22:09



Mathematics, 17.03.2020 22:09


Mathematics, 17.03.2020 22:09

Geography, 17.03.2020 22:09

Mathematics, 17.03.2020 22:09

Mathematics, 17.03.2020 22:09

Mathematics, 17.03.2020 22:09

Chemistry, 17.03.2020 22:09

 molecules are formed.
molecules are formed.
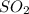 and
and 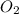 molecules will incraese which in turn will lead to formatioon of more
molecules will incraese which in turn will lead to formatioon of more 


