
Chemistry, 19.12.2020 01:00 nshadow2920
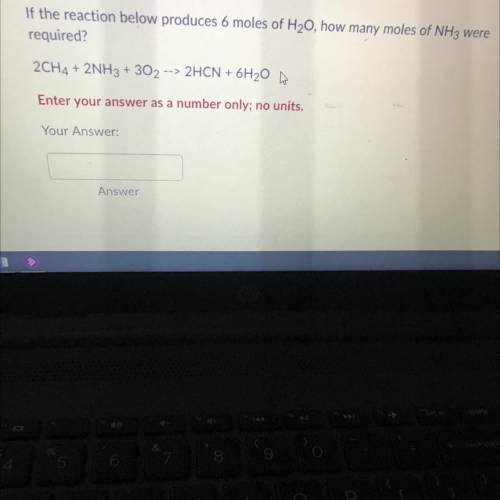
If the reaction below produces 6 moles of H20, how many moles ofNH3 were required?2CH4 + 2NH3 + 302 --> 2HCN + 6H20 m | Enter your answer as a number only; no units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
If the reaction below produces 6 moles of H20, how many moles ofNH3 were required?2CH4 + 2NH3 + 302...
Questions

History, 12.12.2019 04:31

Biology, 12.12.2019 04:31


History, 12.12.2019 04:31

Biology, 12.12.2019 04:31

Mathematics, 12.12.2019 04:31

History, 12.12.2019 04:31

Mathematics, 12.12.2019 04:31

Chemistry, 12.12.2019 04:31

English, 12.12.2019 04:31

Biology, 12.12.2019 04:31


Chemistry, 12.12.2019 04:31

Mathematics, 12.12.2019 04:31


Chemistry, 12.12.2019 04:31

History, 12.12.2019 04:31


Geography, 12.12.2019 04:31



