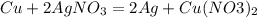When a small piece of copper metal is added to a silver nitrate solution, the following reaction occurs:
2Ag+NO3+Cu → Cu (NO3)2+2Ag
This equation both represents both a single replacement reaction AND a(n) reaction.
A. combustion
B. decomposing
C. neutralization
D. oxidation-reduction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
When a small piece of copper metal is added to a silver nitrate solution, the following reaction occ...
Questions



Computers and Technology, 29.02.2020 03:20




Biology, 29.02.2020 03:20








Mathematics, 29.02.2020 03:20