GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Ques...

Chemistry, 18.12.2020 04:00 sabahtramirez01
GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Question :
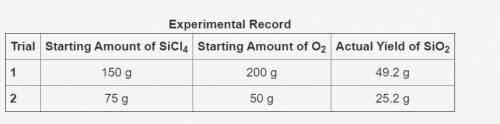
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
The table shows an experimental record for the above reaction.
-> THE TABLE IS IN THE IMAGE <-
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3
You know the right answer?
Questions

History, 06.10.2019 19:30


History, 06.10.2019 19:30


Arts, 06.10.2019 19:30



Advanced Placement (AP), 06.10.2019 19:30

Arts, 06.10.2019 19:30


History, 06.10.2019 19:30


Social Studies, 06.10.2019 19:30

Biology, 06.10.2019 19:30

English, 06.10.2019 19:30


Mathematics, 06.10.2019 19:30


Mathematics, 06.10.2019 19:30

Computers and Technology, 06.10.2019 19:30



