Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
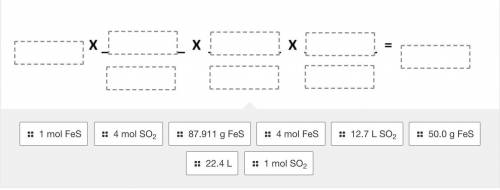
Use the balanced equation given below to drag and drop the terms to correctly solve the following pr...
Questions

Mathematics, 29.01.2020 03:47

Social Studies, 29.01.2020 03:47


Geography, 29.01.2020 03:47

Social Studies, 29.01.2020 03:47

Social Studies, 29.01.2020 03:47


Physics, 29.01.2020 03:47

Arts, 29.01.2020 03:47

History, 29.01.2020 03:47

Mathematics, 29.01.2020 03:47


English, 29.01.2020 03:47

Mathematics, 29.01.2020 03:47



Mathematics, 29.01.2020 03:47

Mathematics, 29.01.2020 03:47