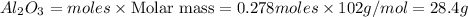Chemistry, 17.12.2020 09:50 xochitlbmartine
Use the reaction given below to solve the problem that follows: Calculate the mass in grams of aluminum oxide produced by the reaction of 15.0 g of aluminum metal.
[ ]grams Al2O3
4 Al + 3 O2 --> 2 Al2O3
**Your answer should be written as XX. X

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Use the reaction given below to solve the problem that follows: Calculate the mass in grams of alumi...
Questions

History, 08.04.2021 16:50

Mathematics, 08.04.2021 16:50








History, 08.04.2021 16:50


Mathematics, 08.04.2021 16:50


Computers and Technology, 08.04.2021 16:50


Mathematics, 08.04.2021 16:50

History, 08.04.2021 16:50

Mathematics, 08.04.2021 16:50

Biology, 08.04.2021 16:50

Mathematics, 08.04.2021 16:50

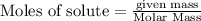
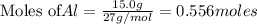
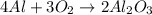
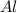 produce == 2 moles of
produce == 2 moles of 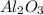
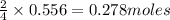 of
of