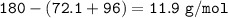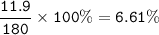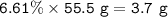Chemistry, 17.12.2020 03:00 azibur3191
Examination reveals that 180.2 g/mol of glucose contains 72.1 grams of carbon, 96 g/mol of oxygen and the remainder is hydrogen. How many g of hydrogen are there in 55.5 g of glucose?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Examination reveals that 180.2 g/mol of glucose contains 72.1 grams of carbon, 96 g/mol of oxygen an...
Questions



Spanish, 30.03.2020 18:57






Mathematics, 30.03.2020 18:57

Biology, 30.03.2020 18:57


Mathematics, 30.03.2020 18:57

Mathematics, 30.03.2020 18:57



Chemistry, 30.03.2020 18:57




History, 30.03.2020 18:57