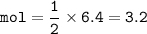Chemistry, 17.12.2020 02:40 leeney6166
Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely with 6.4 moles of lithium hydroxide?
LiOH + H2SO4yields Li2SO4 + H2O (2 points)
Group of answer choices
12.8 mol H2SO4
6.4 mol H2SO4
3.2 mol H2SO4
2.1 mol H2SO4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely wi...
Questions

English, 24.02.2021 02:50

Chemistry, 24.02.2021 02:50

Mathematics, 24.02.2021 02:50

Mathematics, 24.02.2021 02:50






Mathematics, 24.02.2021 02:50

Mathematics, 24.02.2021 02:50


History, 24.02.2021 02:50

English, 24.02.2021 02:50

History, 24.02.2021 02:50

Social Studies, 24.02.2021 02:50




Mathematics, 24.02.2021 02:50