Matter and Energy: Tutorial
Question
Activity
Part A
Each shape in the chart repr...

Matter and Energy: Tutorial
Question
Activity
Part A
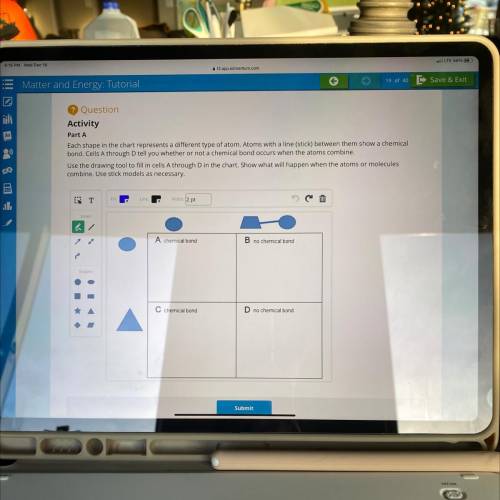
Each shape in the chart represents a different type of atom. Atoms with a line (stick) between them show a chemical
bond. Cells A through D tell you whether or not a chemical bond occurs when the atoms combine.
Use the drawing tool to fill in cells A through D in the chart. Show what will happen when the atoms or molecules
combine. Use stick models as necessary.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Questions


Geography, 18.09.2019 20:30






Physics, 18.09.2019 20:30




Mathematics, 18.09.2019 20:30


Geography, 18.09.2019 20:30



Social Studies, 18.09.2019 20:30


Biology, 18.09.2019 20:30

Mathematics, 18.09.2019 20:30



