Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
...

Chemistry, 16.12.2020 23:00 JoshuaXYP9978
Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
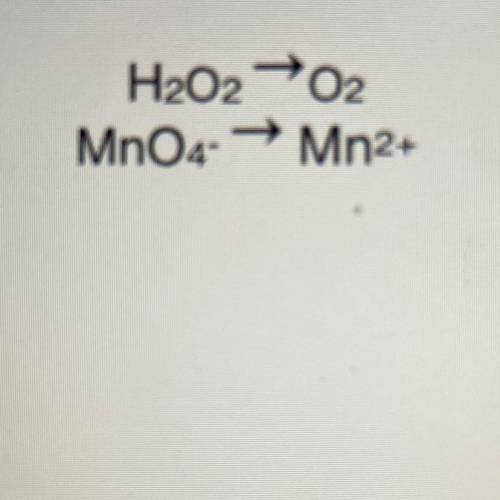

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
Questions

Computers and Technology, 07.10.2019 21:00


Health, 07.10.2019 21:00


English, 07.10.2019 21:00


English, 07.10.2019 21:00


Mathematics, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00

Arts, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

German, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00



Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00



