
Chemistry, 16.12.2020 19:30 hamilclips1748
Moles CO
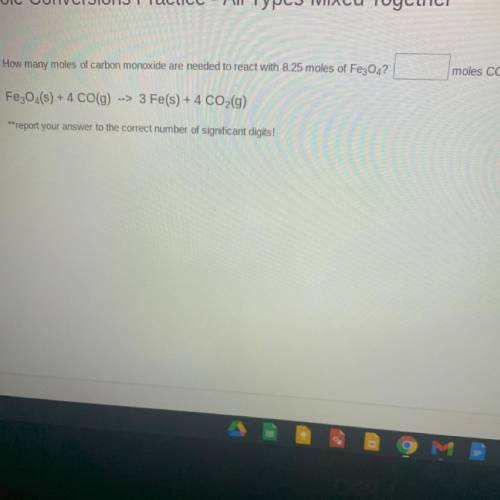
How many moles of carbon monoxide are needed to react with 8.25 moles of Fe3O4?
Fe3O4(s) + 4 CO(9)
--> 3 Fe(s) + 4 CO2(g)
**report your answer to the correct number of significant digits!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2


Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Moles CO
How many moles of carbon monoxide are needed to react with 8.25 moles of Fe3O4?
Fe3O...
Fe3O...
Questions



Social Studies, 04.07.2019 02:20



Mathematics, 04.07.2019 02:30


Geography, 04.07.2019 02:30





Mathematics, 04.07.2019 02:30

Mathematics, 04.07.2019 02:30

History, 04.07.2019 02:30


Biology, 04.07.2019 02:30


History, 04.07.2019 02:30




