
Chemistry, 16.09.2019 13:30 matiasnahuel1011
What is the maximum amount in moles of p2o5 that can theoretically be made from 186 g of p4 and excess oxygen?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1


Chemistry, 23.06.2019 10:30
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2
You know the right answer?
What is the maximum amount in moles of p2o5 that can theoretically be made from 186 g of p4 and exce...
Questions



Mathematics, 29.06.2020 01:01





Mathematics, 29.06.2020 01:01



Mathematics, 29.06.2020 01:01


Mathematics, 29.06.2020 01:01

Biology, 29.06.2020 01:01


Mathematics, 29.06.2020 01:01

Mathematics, 29.06.2020 01:01


Mathematics, 29.06.2020 01:01

English, 29.06.2020 01:01

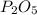 is, 3 moles
is, 3 moles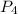 = 186 g
= 186 g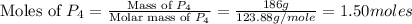
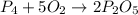
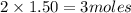 of
of 

