
Chemistry, 16.12.2020 16:50 fansofboys
Calculate the number of milliliters of 0.440 M KOH required to precipitate all of the Fe2+ ions in 187 mL of 0.692 M FeSO4 solution as Fe(OH)2. The equation for the reaction is: FeSO4(aq) + 2KOH(aq) Fe(OH)2(s) + K2SO4(aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Calculate the number of milliliters of 0.440 M KOH required to precipitate all of the Fe2+ ions in 1...
Questions

Health, 27.10.2021 21:20



Mathematics, 27.10.2021 21:20

Mathematics, 27.10.2021 21:20


English, 27.10.2021 21:20



History, 27.10.2021 21:30

Mathematics, 27.10.2021 21:30



Mathematics, 27.10.2021 21:30


English, 27.10.2021 21:30



History, 27.10.2021 21:30

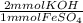 = 258.808 mmol KOH
= 258.808 mmol KOH


