
Chemistry, 16.12.2020 14:00 lesliealaves16
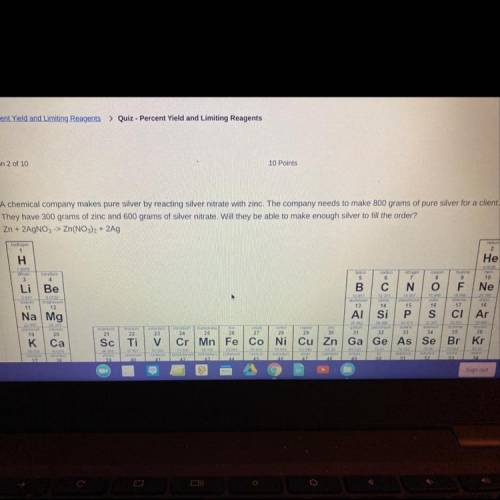
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make s00 grams of pure silver for a client.
They have 300 grams of zinc and 600 grams of silver nitrate. Will they be able to make enough silver to fil the order?
Zn + 2AgNO,> Zn(NO), + 2Ag A. Yes, there will be extra left over. B. Yes, they will have exactly enough C. No, the silver nitrate will run out first. D. No, the zinc nitrate will run out first

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make...
Questions


History, 20.09.2020 04:01


History, 20.09.2020 04:01

English, 20.09.2020 04:01

Social Studies, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01

Spanish, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01




Mathematics, 20.09.2020 04:01

Biology, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01



