
Chemistry, 16.12.2020 14:00 ashvinmsingh
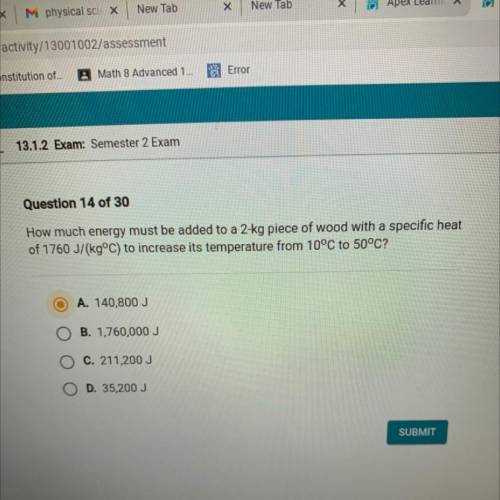
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to increase its temperature from 10°C to 50°C?
A. 140,800 J
B. 1,760,000 J
C. 211,200 J
D. 35,200 J

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to incr...
Questions



Health, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01

Geography, 20.09.2020 19:01




Mathematics, 20.09.2020 19:01

Physics, 20.09.2020 19:01

Physics, 20.09.2020 19:01

Mathematics, 20.09.2020 19:01



Mathematics, 20.09.2020 19:01

English, 20.09.2020 19:01


Social Studies, 20.09.2020 19:01


Mathematics, 20.09.2020 19:01



